Why is ocean water salty?
That is the question everyone asks!
If you have already swallowed water while swimming in the ocean, you must have noticed that seawater is very salty. Did you know that there are approximately 35g of salt in a litre of seawater and less than 1g in a litre of freshwater? So, how did salt manage to end up in sea and ocean waters? In this article, we will explain to you why seawater is salted and why its level of salinity has not changed for millions of years.
Where does ocean salt come from?
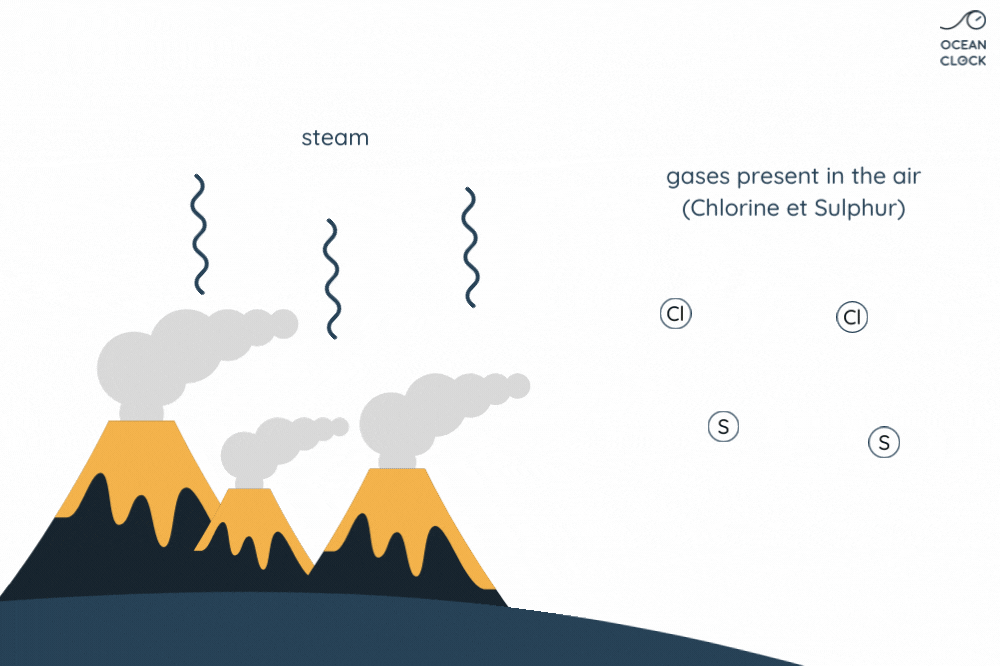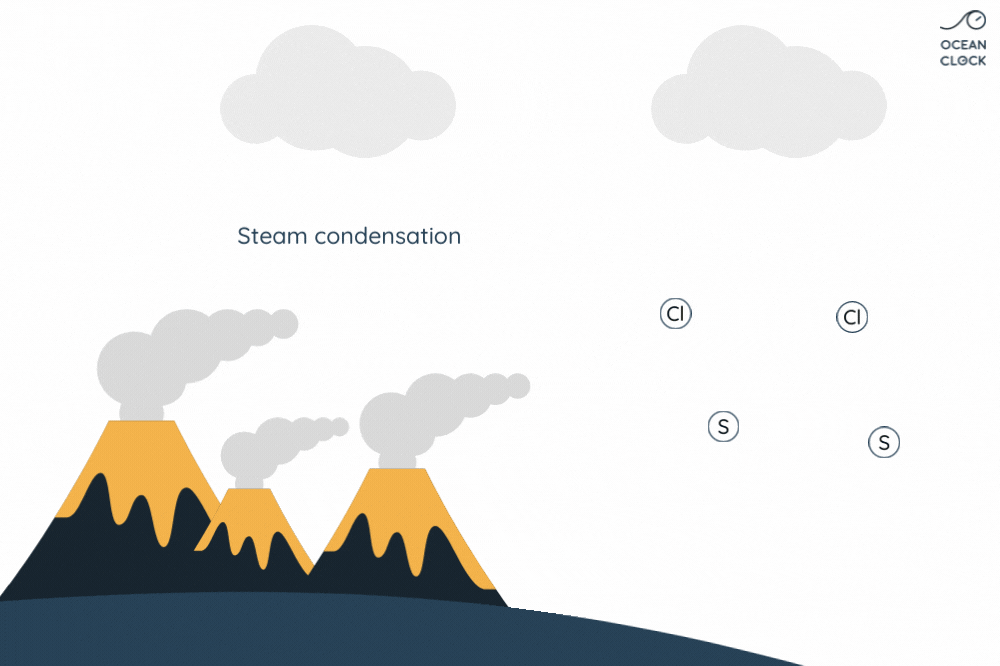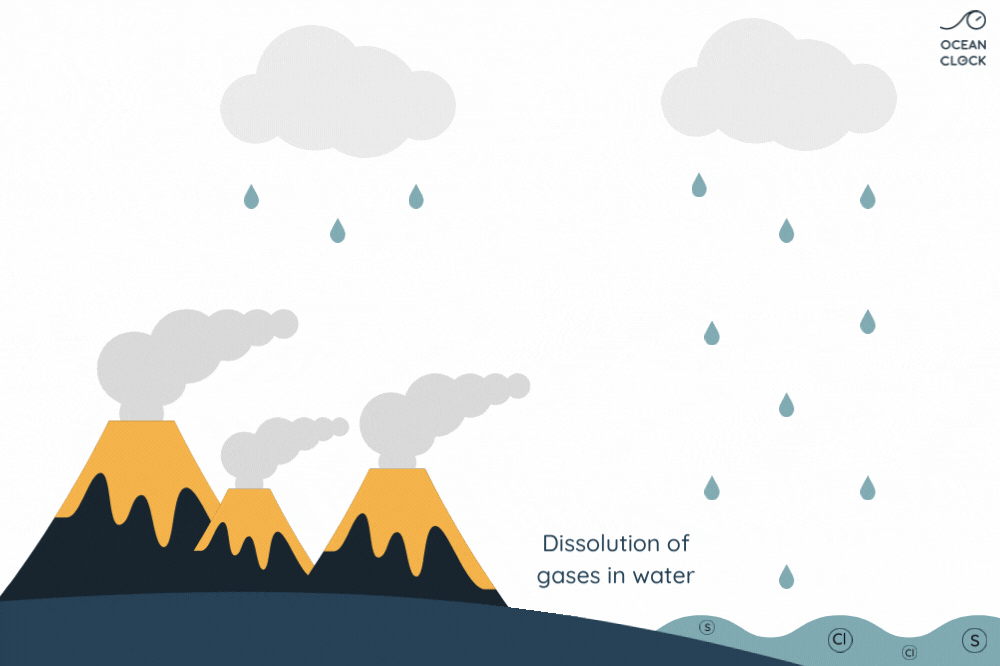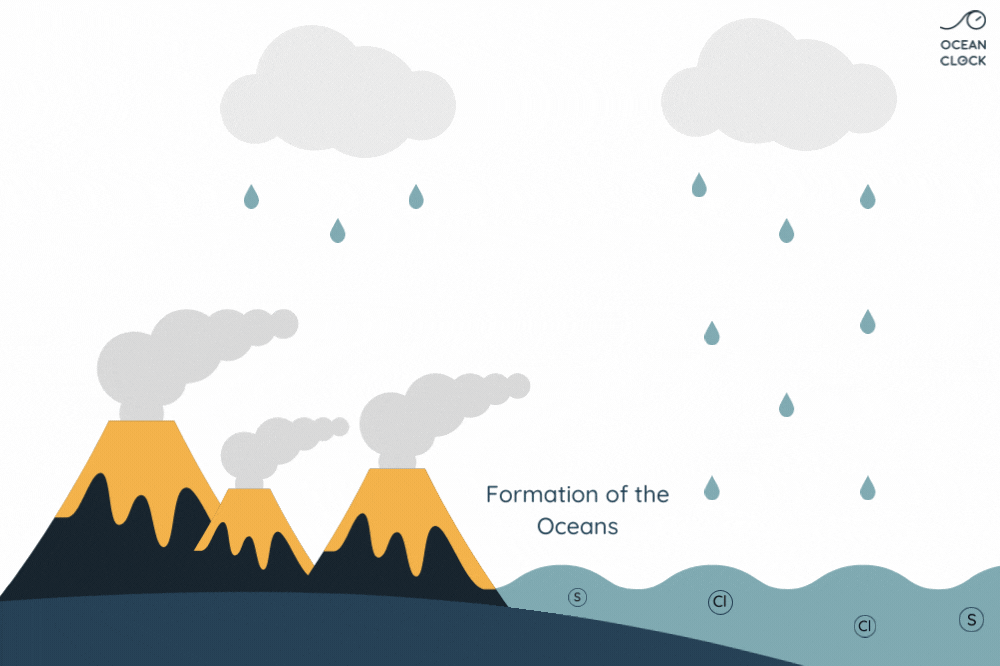
Everything began about 4 billion years ago when oceans first formed. At that time, the planet was covered with active volcanoes but no bodies of water existed.
The numerous active volcanoes on Earth release steam and gases into the atmosphere. This steam condenses into droplets which turn into clouds, and that is how water cycle begins. Once they get bigger, these droplets break away from clouds and thus create rainfalls which result in forming oceans. When in contact with rain, the gases present in the air and which contain sulphur and chlorine, dissolve in water. It is the chlorine that salts oceans.
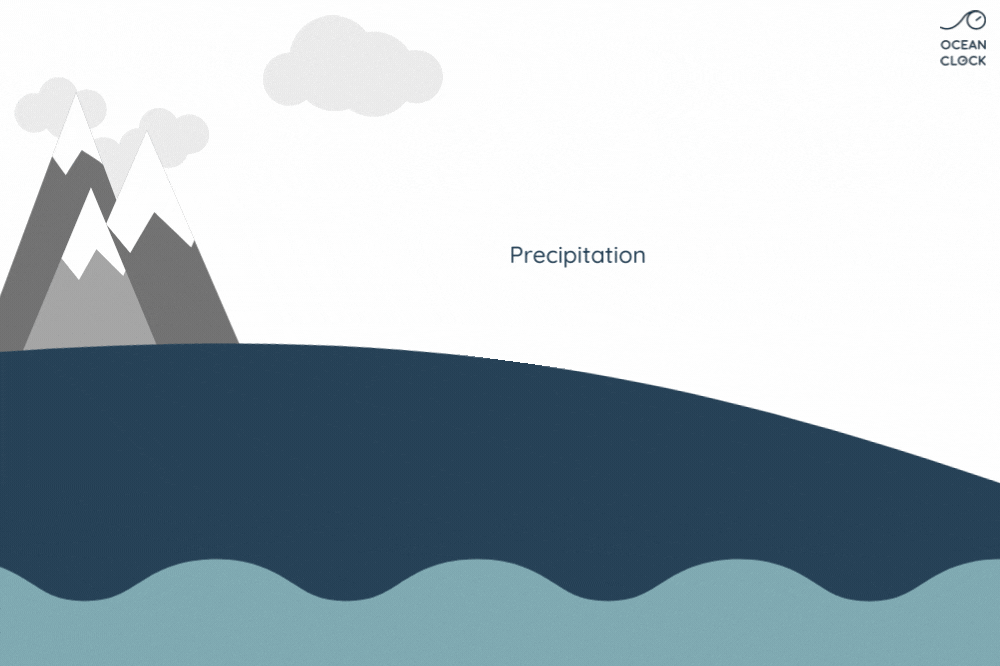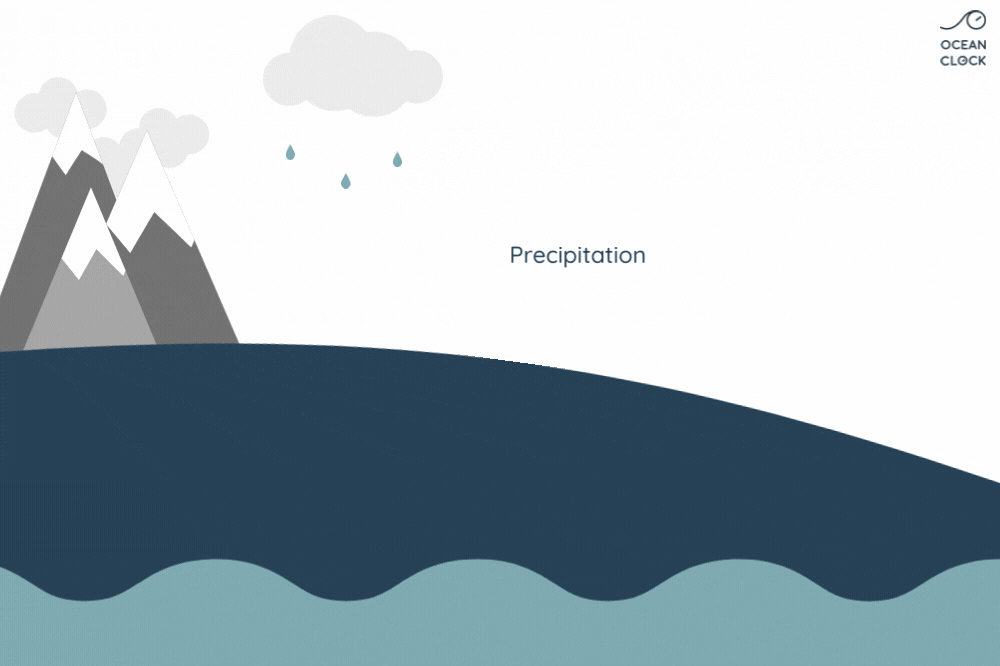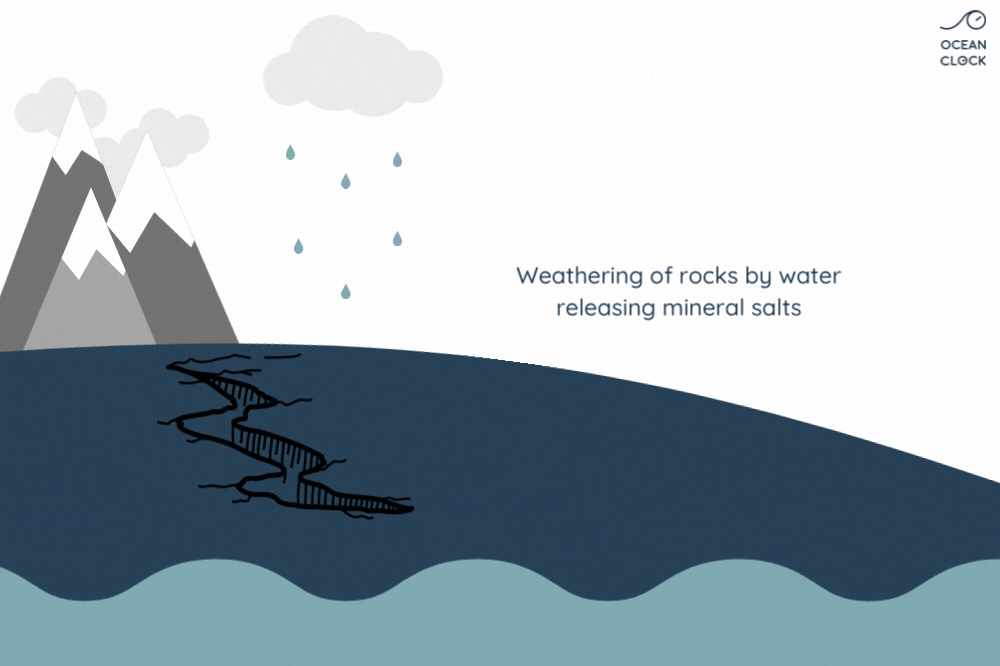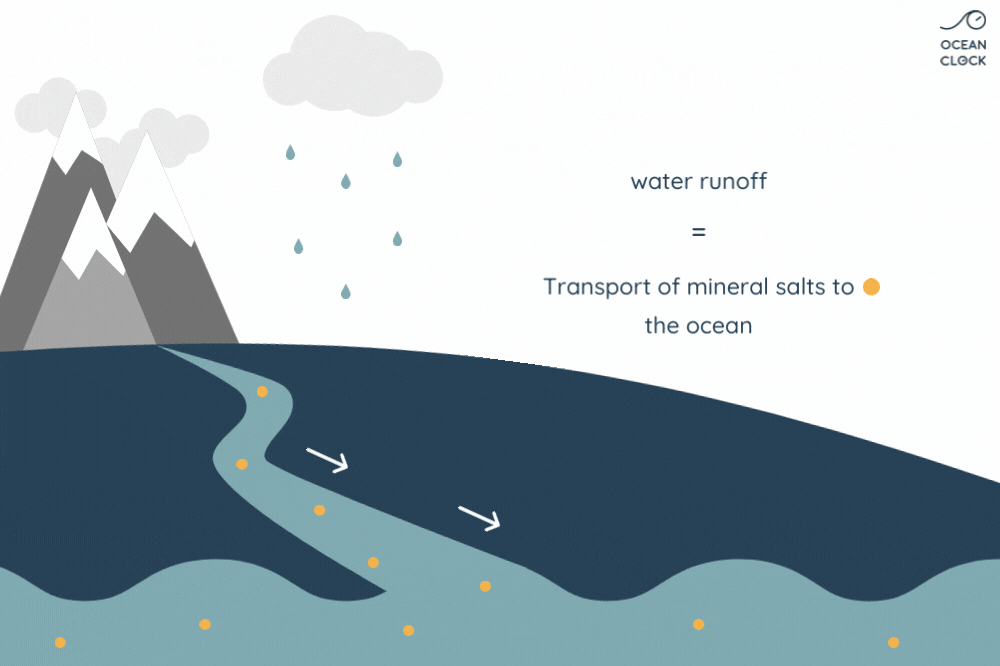
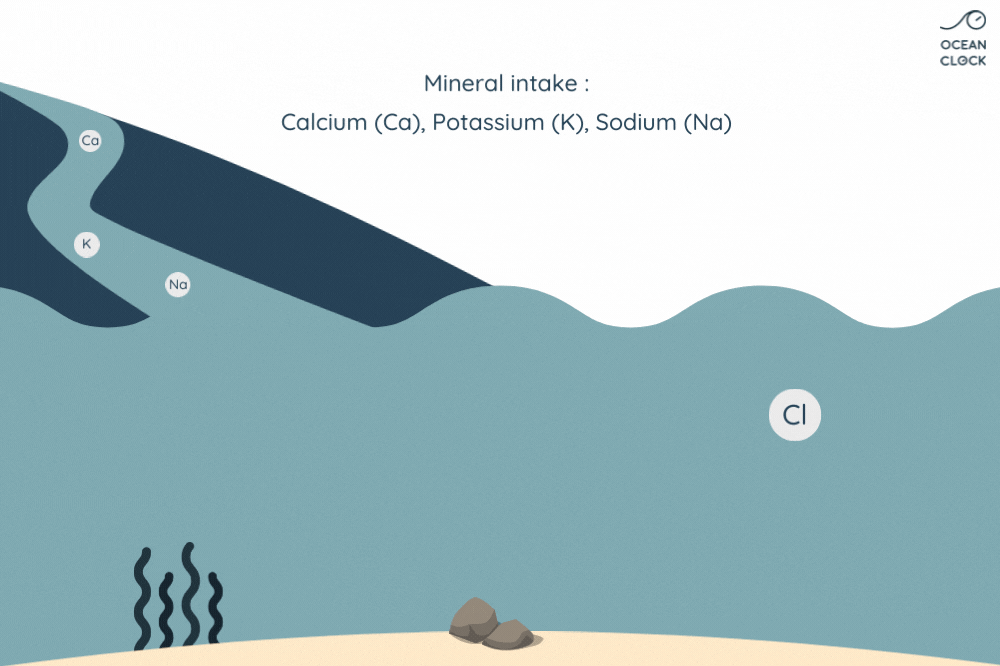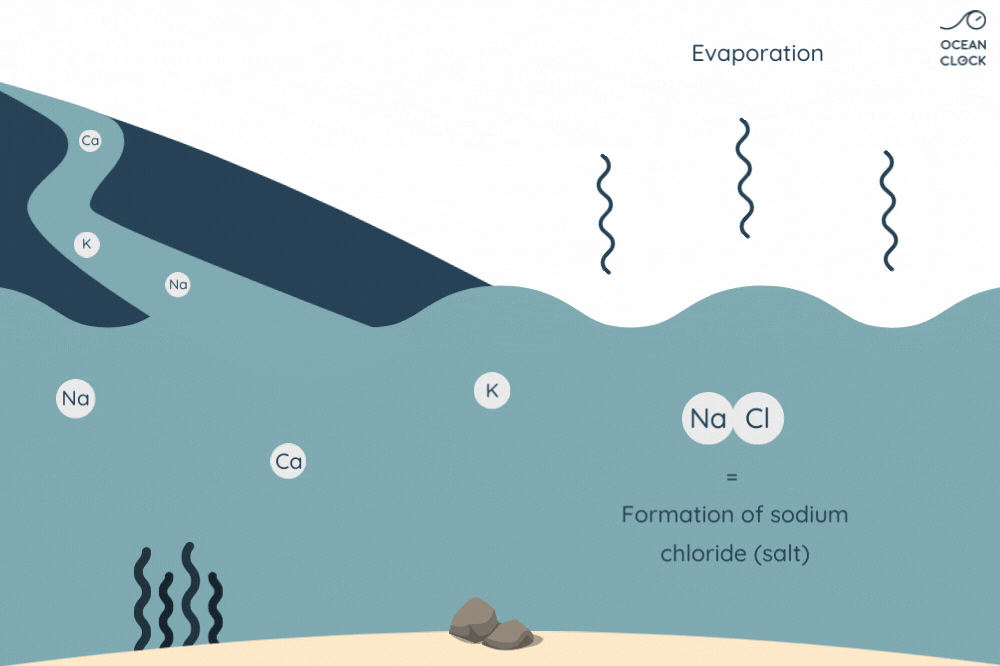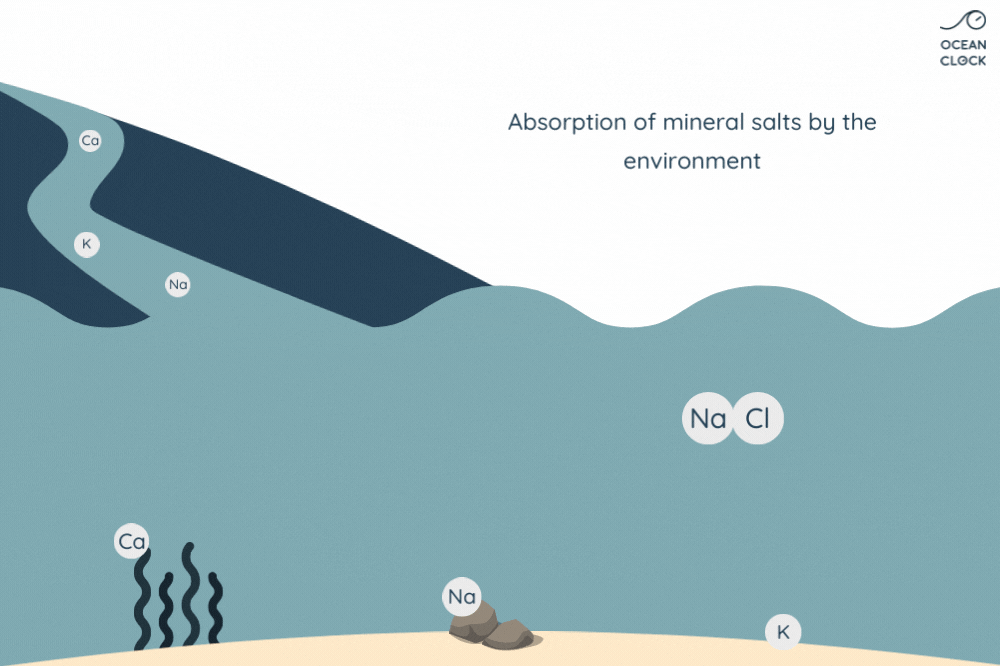
With erosion, other mineral salts are added to chlorine, which results in increasing the oceans’ salinity level. Minerals present on rocks such as sodium, magnesium, potassium and calcium, fall off because of rainwater runoff and are then carried to oceans.
It is when water evaporates that chlorine and sodium come apart to form sodium chloride, that is to say ocean and sea salt. Even if other mineral salts are present in oceans, sodium chloride represents 78% of oceans’ salt. This is the salt we actually put in our food. For information, it is the chloride that gives the salty taste to seawater whereas sodium makes our tastebuds sensitive.
Why doesn’t oceans’ salinity level increase?
Thanks to the water cycle, we know that mineral salts keep getting into oceans. Ocean water evaporates under the effect of heat to form clouds. However, unlike water, salt does not evaporate and thus increases the content of sodium chloride in oceans. It is therefore logical to ask ourselves why seawater salinity has not changed for millions of years. So, how come the oceans’ salt content remains stable?
There are indeed explanations to why the level of salinity of oceans doesn’t increase. Remember our article on sea currents where we explained thermohaline circulation. As you might have read in this article, ocean water keeps moving all around the planet, notably at surface thanks to both the wind and the Earth’s rotation, but also at depth due to water temperature changes as well as salt content changes.
Sea currents enable to transport salt to areas where salinity is lower. This low level of salinity can be explained by the fact that larger amounts of freshwater are added due to heavy rainfalls or to ocean areas where big rivers flow into.
Be aware that every year, waterways carry more than 2 billion tons of rainwater to oceans. While running through small and big rivers, the rainwater carries other mineral salts that end up mixing with sodium chloride that is already present in the ocean. However, these minerals do not have a strong impact on ocean water salinity as they get absorbed along the way by the environment. Indeed, calcium is absorbed by marine life, potassium by the clay present on seabed, and a part of sodium by the ocean floor’s volcanic rocks.
Basically, salt intake is offset by its absorption, which explains why ocean water has been able to keep its level of salinity stable for so many years.
Want to bring a bit of ocean into your home interior? Discover our modern and stylish tide clocks so you can keep an eye on the tide of your favourite beach.


